Clinical Guideline
Injectable HPC is recommended by the SMFM to reduce the risk of preterm birth for clinically indicated patients2
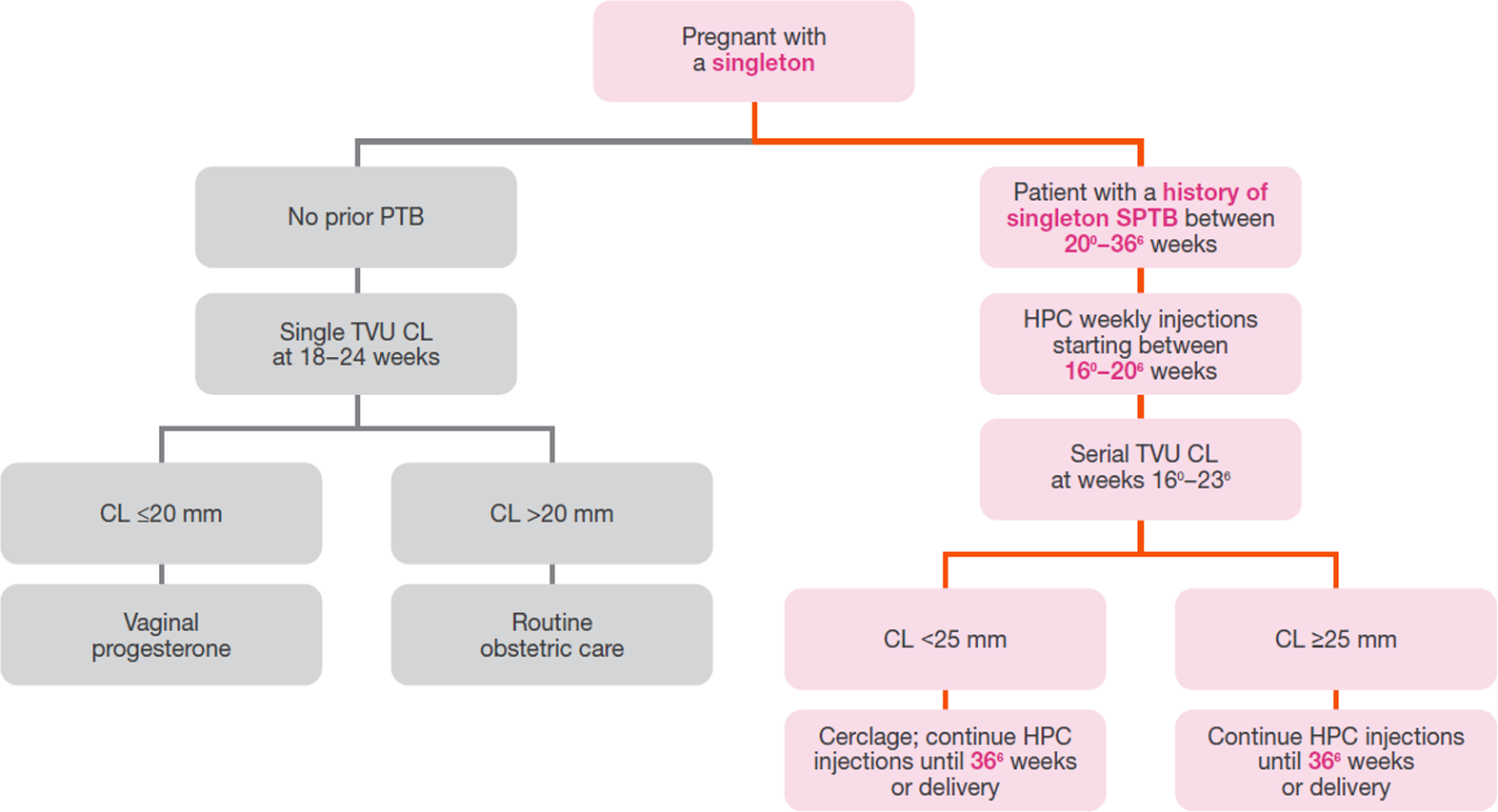
Per the 2017 SMFM statement on choice of progestogen, vaginal progesterone should not be considered a substitute for HPC in these at‑risk women.3
Adapted from the SMFM Clinical Guideline published in the May 2012 American Journal of Obstetrics & Gynecology.2
Emphasis added to highlight patient identification and initiation, and duration of therapy.
HPC=hydroxyprogesterone caproate; SMFM=Society for Maternal-Fetal Medicine; PTB=preterm birth; SPTB=spontaneous preterm birth; TVU=transvaginal ultrasound; CL=cervical length.
In a clinical study, comparable bioavailability was seen between Makena Auto-Injector dosed subcutaneously (1.1 mL; back of upper arm) and Makena dosed IM (1 mL; gluteus maximus).1*
*In a single-dose, open-label, randomized, parallel design bioavailability study in 120 healthy post-menopausal women.

